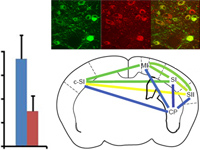
Optogenetics provides a means to dissect the organization and function of neural circuits. Optogenetics also offers the translational promise of restoring sensation, enabling movement or supplanting abnormal activity patterns in pathological brain circuits. However, the inherent sluggishness of evoked photocurrents in conventional channelrhodopsins has hampered the development of optoprostheses that adequately mimic the rate and timing of natural spike patterning. Here, we explore the feasibility and limitations of a central auditory optoprosthesis by photoactivating mouse auditory midbrain neurons that either express channelrhodopsin-2 (ChR2) or Chronos, a channelrhodopsin with ultra-fast channel kinetics. Chronos-mediated spike fidelity surpassed ChR2 and natural acoustic stimulation to support a superior code for the detection and discrimination of rapid pulse trains. Interestingly, this midbrain coding advantage did not translate to a perceptual advantage, as behavioral detection of midbrain activation was equivalent with both opsins. Auditory cortex recordings revealed that the precisely synchronized midbrain responses had been converted to a simplified rate code that was indistinguishable between opsins and less robust overall than acoustic stimulation. These findings demonstrate the temporal coding benefits that can be realized with next-generation channelrhodopsins, but also highlight the challenge of inducing variegated patterns of forebrain spiking activity that support adaptive perception and behavior.