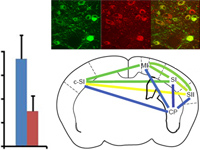
Optogenetic methods have been highly effective for suppressing neural activity and modulating behavior in rodents, but effects have been much smaller in primates, which have much larger brains. Here, we present a suite of technologies to use optogenetics effectively in primates and apply these tools to a classic question in oculomotor control. First, we measured light absorption and heat propagation in vivo, optimized the conditions for using the red-light–shifted halorhodopsin Jaws in primates, and developed a large-volume illuminator to maximize light delivery with minimal heating and tissue displacement. Together, these advances allowed for nearly universal neuronal inactivation across more than 10 mm3 of the cortex. Using these tools, we demonstrated large behavioral changes (i.e., up to several fold increases in error rate) with relatively low light power densities (≤100 mW/mm2) in the frontal eye field (FEF). Pharmacological inactivation studies have shown that the FEF is critical for executing saccades to remembered locations. FEF neurons increase their firing rate during the three epochs of the memory-guided saccade task: visual stimulus presentation, the delay interval, and motor preparation. It is unclear from earlier work, however, whether FEF activity during each epoch is necessary for memory-guided saccade execution. By harnessing the temporal specificity of optogenetics, we found that FEF contributes to memory-guided eye movements during every epoch of the memory-guided saccade task (the visual, delay, and motor periods).