Technologies for silencing the electrical activity of genetically targeted neurons
in the brain are
important for assessing the contribution of specific cell types and pathways toward
behaviors
and pathologies. Recently we found that archaerhodopsin-3 from Halorubrum sodomense
(Arch), a light-driven outward proton pump, when genetically expressed in neurons,
enables
them to be powerfully, transiently, and repeatedly silenced in response to pulses of
light.
Because of the impressive characteristics of Arch, we explored the optogenetic
utility of opsins
with high sequence homology to Arch, from archaea of the Halorubrum genus. We found
that
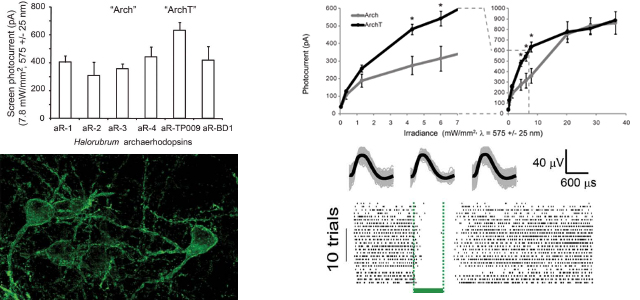
the archaerhodopsin from Halorubrum strain TP009, which we named ArchT, could
mediate
photocurrents of similar maximum amplitude to those of Arch (~900 pA in vitro), but
with a
>3-fold improvement in light sensitivity over Arch, most notably in the optogenetic
range of
1–10 mW/mm2, equating to >2x increase in brain tissue volume addressed by a typical
single
optical fiber. Upon expression in mouse or rhesus macaque cortical neurons, ArchT
expressed
well on neuronal membranes, including excellent trafficking for long distances down
neuronal
axons. The high light sensitivity prompted us to explore ArchT use in the cortex of
the rhesus
macaque. Optical perturbation of ArchT-expressing neurons in the brain of an awake
rhesus
macaque resulted in a rapid and complete (~100%) silencing of most recorded cells,
with
suppressed cells achieving a median firing rate of 0 spikes/s upon illumination. A
small population
of neurons showed increased firing rates at long latencies following the onset of
light stimulation,
suggesting the existence of a mechanism of network-level neural activity balancing.
The powerful
net suppression of activity suggests that ArchT silencing technology might be of
great use not
only in the causal analysis of neural circuits, but may have therapeutic
applications.