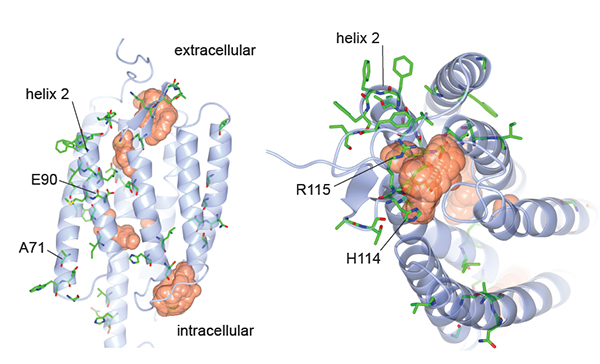
Channelrhodopsins (ChRs) are light-gated ion channels in widespread use in neuroscience for mediating the genetically targetable optical control of neurons (optogenetics). Wild-type ChRs pass multiple kinds of ions, and although nonspecific ChR-mediated conductance is not an issue in many neuroscience studies, conductance of calcium and protons, which can mediate diverse cellular signals, may be undesirable in some instances. We here turned our attention to the creation of ChRs that have high cation photocurrent, but pass fewer calcium ions and protons. We developed an automated, time-resolved screening method capable of rapidly phenotyping channelrhodopsin-2 (ChR2) variants. We found substitution mutations throughout ChR2 that could boost current while altering ion selectivity, and also observed that the mutations that reduced calcium or proton conductance have additive effects. By combining four mutations, we obtained a ChR, ChromeQ, with improved photocurrent that possesses order-of-magnitude reductions in calcium and proton conductance and high fidelity in driving repetitive action potentials in neurons. The approach presented here offers a viable pathway toward customization of complex physiological properties of optogenetic tools. We propose that our screening method not only enables elucidation of new ChR variants that affect microbial opsin performance, but may also reveal new principles of optogenetic protein engineering.