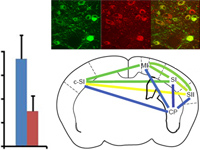
The mechanisms that enable the hippocampal network to express the appropriate spatial representation for a particular circumstance are not well understood. Previous studies suggest that the medial entorhinal cortex (MEC) may have a role in reproducibly selecting the hippocampal representation of an environment. To examine how ongoing MEC activity is continually integrated by the hippocampus, we performed transient unilateral optogenetic inactivations of the MEC while simultaneously recording place cell activity in CA1. Inactivation of the MEC caused a partial remapping in the CA1 population, without diminishing the degree of spatial tuning across the active cell assembly. These changes remained stable irrespective of intermittent disruption of MEC input, indicating that while MEC input is integrated over long time scales to bias the active population, there are mechanisms for stabilizing the population of active neurons independent of the MEC. We find that MEC inputs to the hippocampus shape its ongoing activity by biasing the participation of the neurons in the active network, thereby influencing how the hippocampus selectively represents information.